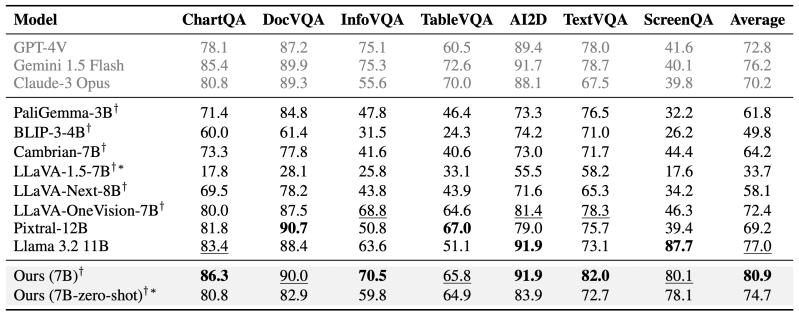
It has been a grim few months for the Duchenne muscular dystrophy (DMD) community. There had been some excitement when, a couple of years ago, a gene therapy for the disorder was approved by the US Food and Drug Administration for the first time. That drug, Elevidys, has now been implicated in the deaths of two teenage boys.
The drug’s approval was always controversial—there was a lack of evidence that it actually worked, for starters. But the agency that once rubber-stamped the drug has now turned on its manufacturer, Sarepta Therapeutics. In a remarkable chain of events, the FDA asked the company to stop shipping the drug on July 18. Sarepta refused to comply.
In the days since, the company has acquiesced. But its reputation has already been hit. And the events have dealt a devastating blow to people desperate for treatments that might help them, their children, or other family members with DMD.
DMD is a rare genetic disorder that causes muscles to degenerate over time. It’s caused by a mutation in a gene that codes for a protein called dystrophin. That protein is essential for muscles—without it, muscles weaken and waste away. The disease mostly affects boys, and symptoms usually start in early childhood.
At first, affected children usually start to find it hard to jump or climb stairs. But as the disease progresses, other movements become difficult too. Eventually, the condition might affect the heart and lungs. The life expectancy of a person with DMD has recently improved, but it is still only around 30 or 40 years. There is no cure. It’s a devastating diagnosis.
Elevidys was designed to replace missing dystrophin with a shortened, engineered version of the protein. In June 2023, the FDA approved the therapy for eligible four- and five-year-olds. It came with a $3.2 million price tag.
The approval was celebrated by people affected by DMD, says Debra Miller, founder of CureDuchenne, an organization that funds research into the condition and offers support to those affected by it. “We’ve not had much in the way of meaningful therapies,” she says. “The excitement was great.”
But the approval was controversial. It came under an “accelerated approval” program that essentially lowers the bar of evidence for drugs designed to treat “serious or life-threatening diseases where there is an unmet medical need.”
Elevidys was approved because it appeared to increase levels of the engineered protein in patients’ muscles. But it had not been shown to improve patient outcomes: It had failed a randomized clinical trial.
The FDA approval was granted on the condition that Sarepta complete another clinical trial. The topline results of that trial were described in October 2023 and were published in detail a year later. Again, the drug failed to meet its “primary endpoint”—in other words, it didn’t work as well as hoped.
In June 2024, the FDA expanded the approval of Elevidys. It granted traditional approval for the drug to treat people with DMD who are over the age of four and can walk independently, and another accelerated approval for those who can’t.
Some experts were appalled at the FDA’s decision—even some within the FDA disagreed with it. But things weren’t so simple for people living with DMD. I spoke to some parents of such children a couple of years ago. They pointed out that drug approvals can help bring interest and investment to DMD research. And, above all, they were desperate for any drug that might help their children. They were desperate for hope.
Unfortunately, the treatment does not appear to be delivering on that hope. There have always been questions over whether it works. But now there are serious questions over how safe it is.
In March 2025, a 16-year-old boy died after being treated with Elevidys. He had developed acute liver failure (ALF) after having the treatment, Sarepta said in a statement. On June 15, the company announced a second death—a 15-year-old who also developed ALF following Elevidys treatment. The company said it would pause shipments of the drug, but only for patients who are not able to walk.
The following day, Sarepta held an online presentation in which CEO Doug Ingram said that the company was exploring ways to make the treatment safer, perhaps by treating recipients with another drug that dampens their immune systems. But that same day, the company announced that it was laying off 500 employees—36% of its workforce. Sarepta did not respond to a request for comment.
On June 24, the FDA announced that it was investigating the risks of serious outcomes “including hospitalization and death” associated with Elevidys, and “evaluating the need for further regulatory action.”
There was more tragic news on July 18, when there were reports that a third patient had died following a Sarepta treatment. This patient, a 51-year-old, hadn’t been taking Elevidys but was enrolled in a clinical trial for a different Sarepta gene therapy designed to treat limb-girdle muscular dystrophy. The same day, the FDA asked Sarepta to voluntarily pause all shipments of Elevidys. Sarepta refused to do so.
The refusal was surprising, says Michael Kelly, chief scientific officer at CureDuchenne: “It was an unusual step to take.”
After significant media coverage, including reporting that the FDA was “deeply troubled” by the decision and would use its “full regulatory authority,” Sarepta backed down a few days later. On July 21, the company announced its decision to “voluntarily and temporarily” pause all shipments of Elevidys in the US.
Sarepta says it will now work with the FDA to address safety and labeling concerns. But in the meantime, the saga has left the DMD community grappling with “a mix of disappointment and concern,” says Kelly. Many are worried about the risks of taking the treatment. Others are devastated that they are no longer able to access it.
Miller says she knows of families who have been working with their insurance providers to get authorization for the drug. “It’s like the rug has been pulled out from under them,” she says. Many families have no other treatment options. “And we know what happens when you do nothing with Duchenne,” she says. Others, particularly those with teenage children with DMD, are deciding against trying the drug, she adds.
The decision over whether to take Elevidys was already a personal one based on several factors, he says. People with DMD and their families deserve clear and transparent information about the treatment in order to make that decision.
The FDA’s decision to approve Elevidys was made on limited data, says Kelly. But as things stand today, over 900 people have been treated with Elevidys. “That gives the FDA… an opportunity to look at real data and make informed decisions,” he says.
“Families facing Duchenne do not have time to waste,” Kelly says. “They must navigate a landscape where hope is tempered by the realities of medical complexity.”
A version of this article first appeared in The Checkup, MIT Technology Review’s weekly biotech newsletter. To receive it in your inbox every Thursday, and read articles like this first, sign up here.