In association withGlobant
Amid the turbulence of the wider global economy in recent years, the pharmaceuticals industry is weathering its own storms. The rising cost of raw materials and supply chain disruptions are squeezing margins as pharma companies face intense pressure—including from countries like the US—to control drug costs. At the same time, a wave of expiring patents threatens around $300 billion in potential lost sales by 2030. As companies lose the exclusive right to sell the drugs they have developed, competitors can enter the market with generic and biosimilar lower-cost alternatives, leading to a sharp decline in branded drug sales—a “patent cliff.” Simultaneously, the cost of bringing new drugs to market is climbing. McKinsey estimates cost per launch is growing 8% each year, reaching $4 billion in 2022.

In clinics and health-care facilities, norms and expectations are evolving, too. Patients and health-care providers are seeking more personalized services, leading to greater demand for precision drugs and targeted therapies. While proving effective for patients, the complexity of formulating and producing these drugs makes them expensive and restricts their sale to a smaller customer base.
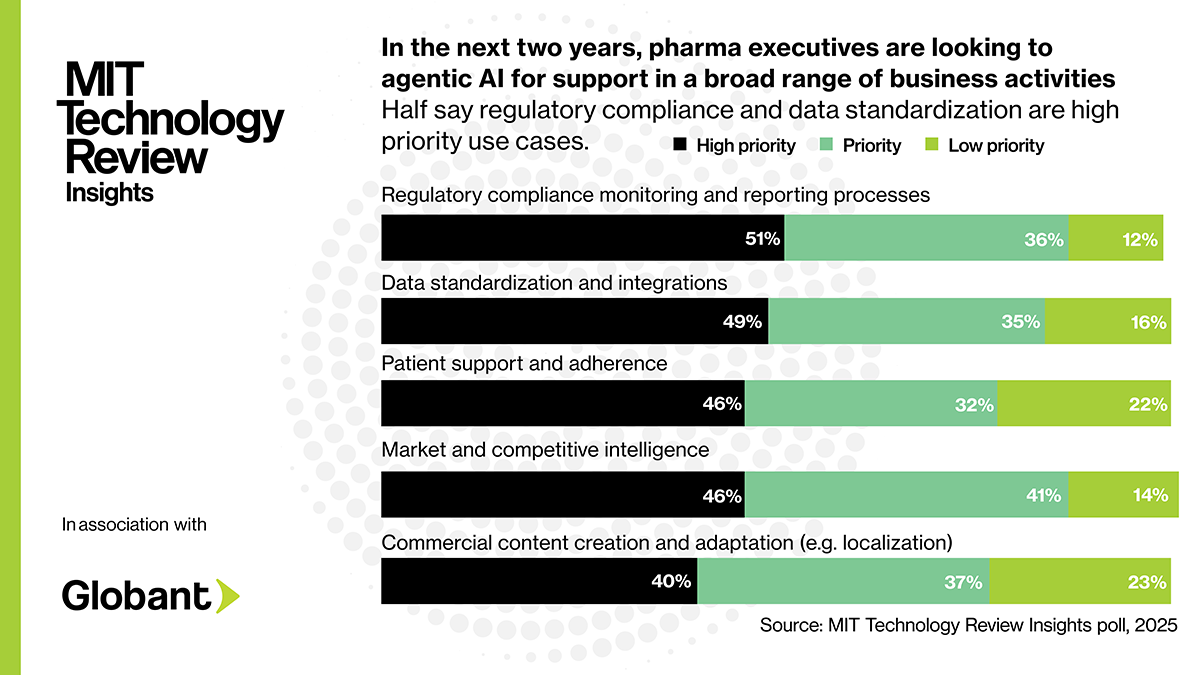
The need for personalization extends to sales and marketing operations too as pharma companies are increasingly needing to compete for the attention of health-care professionals (HCPs). Estimates suggest that biopharmas were able to reach 45% of HCPs in 2024, down from 60% in 2022. Personalization, real-time communication channels, and relevant content offer a way of building trust and reaching HCPs in an increasingly competitive market. But with ever-growing volumes of content requiring medical, legal, and regulatory (MLR) review, companies are struggling to keep up, leading to potential delays and missed opportunities.
This content was produced by Insights, the custom content arm of MIT Technology Review. It was not written by MIT Technology Review’s editorial staff. It was researched, designed, and written by human writers, editors, analysts, and illustrators. AI tools that may have been used were limited to secondary production processes that passed thorough human review.